Dive Brief:
- Moderna on Monday said its vaccine for COVID-19 should still protect against coronavirus variants first detected in the U.K. and in South Africa, citing results from laboratory testing that showed the blood of immunized volunteers in an early study contained antibodies capable of neutralizing the virus.
- Even so, the Cambridge, Massachusetts-based biotech plans to study whether an additional booster shot, or a new booster engineered specifically against the South African variant, could provide better protection.
- Vaccine and public health experts have grown more concerned that accumulating mutations in the spike protein targeted by many of the shots authorized and in testing could help the coronavirus evade vaccination. So far, however, evidence indicates the available vaccines, which generate multifaceted immune responses, should still be effective.
Dive Insight:
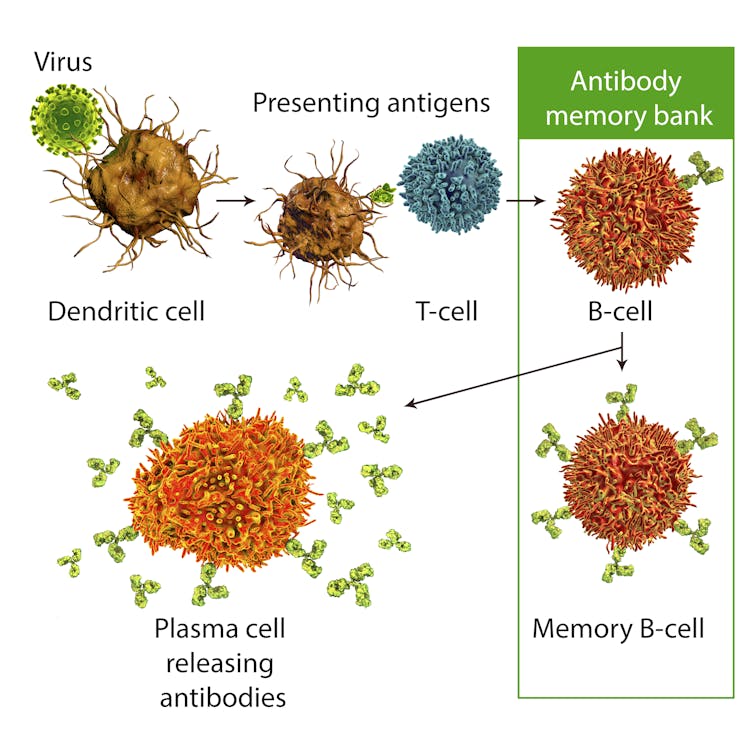
One of the chief advantages of the messenger RNA vaccine technology used by Moderna as well as Pifzer and BioNTech is the ease by which it can be adapted.
At the core of mRNA vaccines is a nucleic acid sequence encoding for a target viral protein, like the coronavirus' characteristic spike. In the case of Moderna's shot, determining the right sequence took researchers at Moderna and the U.S. National Institutes of Health just two days after Chinese researchers first posted the coronavirus genome online. A Phase 1 clinical trial followed roughly 60 days later.
In theory, updating an mRNA vaccine to address new mutations in the target protein should require only tweaks to that sequence, and subsequent testing to ensure the encoded protein still reliably raises a protective immune response.
Moderna's announcement Monday that it will proceed with studies of a re-engineered booster shot is suggestive of the speed at which that first step can be carried out. The company said it would advance the new strain-specific booster into preclinical testing and a Phase 1 study in the U.S.
Company CEO Stéphane Bancel said the decision was made "out of an abundance of caution" following laboratory testing on the two variants. Using blood samples from eight volunteers vaccinated with its shot in an early study, Moderna measured the levels of neutralizing antibodies for the variants originating in the U.K. and South Africa, dubbed B.1.1.7 and B.1.351, respectively.
Emerging data have shown both variants appear to be more infectious, although it's not certain whether they also are more deadly.
In tests against the U.K. variant, there was no significant impact to neutralizing antibody levels compared with prior variants, Moderna said.
Against the South African variant, however, neutralizing antibody levels were six-fold lower. Based on data from testing in monkeys, Moderna believes its shot will still protect people infected with that variant against COVID-19. But the company noted the lower antibody levels "may suggest a potential risk of earlier waning of immunity to the new B.1.351 strains."
A draft manuscript detailing Moderna's findings was posted on the pre-print server bioRxiv Monday.
Pfizer and BioNtech's vaccine, which was shown similarly effective to Moderna's in late-stage trials, also proved effective against the U.K. variant in laboratory tests, the companies said last week.
While mRNA vaccines may prove readily adaptable, scientists are still unsure exactly what levels of antibodies are needed for full protection, potentially complicating efforts to prove updated boosters to regulators.
Peter Marks, the FDA's top official tasked with reviewing vaccines, recently suggested in an interview with WebMD that replicating the large, time-consuming Phase 3 trials initially used to prove vaccine efficacy may not be needed again.
"[T]he first time we do it, we will check immunogenicity," Marks said in the Dec. 29 interview. "But it is not going to have to be another 30,000 patient clinical trial. Those immunogenicity studies are usually 400 patients, just to make sure that we have the right check of what's coming out."
The Link LonkJanuary 25, 2021 at 11:57PM
https://ift.tt/3sVdobl
Moderna to study vaccine booster aimed at coronavirus variant - BioPharma Dive
https://ift.tt/2DVP6sH